Karl Fischer titration and calibration Procedure:
Introduction
Principle of Karl Fischer titration
Karl Fischer titration and calibration Procedure:-
Calibration Operation:
Step by Step guide to calibrating the manual Karl Fischer titrator:
- Prepare the fresh KF reagent as per the SOP for reagent preparation and the manufacturer's manual.
- Weigh the standard Sodium Tartrate Dihydrate accurately, the standard should be certified, and record the weight in the notebook.
- Titration Setup for analysis: Fill the titration cell of the titrator with fresh prepared KF reagent and install the electrodes carefully.
- Run a blank titration without putting standard to titration cell, it will help to determine the background signal or drift.
- Now, add the weighed standard into the titration cell, and titrate until the endpoint is obtained, Record the volume of the KF reagent consumed.
- Then calculate the titer of the KF reagent using the standard's weight and the volume of the KF reagent consumed in the titration.
- Also, calculate the drift correction factor using the blank titration results found before starting the standard's titration.
- Adjust the titer of the KF reagent by multiplying it with the drift correction factor.
- Now, verify the titer adjustment by performing another titration with the same standard. The results should be within the acceptable limit of deviation.
- Record all the calibration data, including the date, the standard used, the volume of KF reagent consumed, the titer, the drift correction factor, and the adjusted titer in the calibration logbook or sheets, do signatures of the test performer chemist, and the Quality Manager who approved the calibration process.
For Solid samples or Raw materials:
“Percentage”, “PPM” and “mg of H2O”.
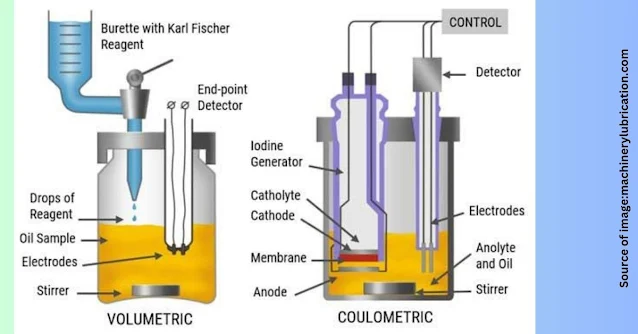
Diagram of Karl Fischer Titrator
Calibration:
List of annexures and formats.
The user manual of Karl Fischer
Top 3 Karl Fischer Titrator used in Pharma Industry Labs.
- Lab Junction Karl Fischer Moisture Titrator, Microprocessor Karl Fischer Moisture Titrator-BUY
- Labgo Auto Karl Fischer 002; See here
- VSI ELECTRONICS PRIVATE LIMITED VSI-71 Microprocessor Karl Fischer Moisture Titrator; Learn more
- Many pharmaceutical products and APIs contain water content in its molecule, which can affect the stability of products, efficacy, and also the shelf-life. To rule out such issues determination of water content in products and API used in the manufacturing of products is carried out. After knowing the exact value of water several factors can be applied to the requisition of Raw materials to overcome the loss of water content during the process and life duration.
- Karl Fischer titrator is an essential instrument in Laboratory according to FDA regulations. It is equally important as UV Visible Spectrophotometer, and HPLC in the laboratory of any manufacturing company.
- Karl Fischer's titrator is also important for process optimization in the company. It can help a manufacturing chemist to calculate the possible loss of mass during the manufacturing process by knowing the actual water content in the API used.
Summary
References
- Water Determination<921>-USP-31
- GR Scientific, 71-0000, Aquamax Coulometric Karl Fischer Titrator-Buy Amazon
Karl Fischer Titration: Determination of Water by Volhard Method, by E. W. Schmid, ISBN 978-3-527-60936-9.-pdf
Karl Fischer Titration: A Practical Approach, by G. G. Guilbault, ISBN 978-0-8247-9753-3.
Metrological Aspects of Karl Fischer Titration, by J. M. Llabrés i Xamena, Journal of Chemical Education, Vol. 77, No. 11, 2000, pp. 1475-1479.
Karl Fischer Titration: Recent Developments and Applications, by J. P. Hartmann and K. D. Luong, Analytical Chemistry, Vol. 65, No. 12, 1993, pp. 707A-712A.
Calibration of Karl Fischer Titrators: A Review, by G. G. Guilbault, Analytica Chimica Acta, Vol. 288, No. 2, 1994, pp. 173-181.
The Use of Gravimetric Standards for the Calibration of Karl Fischer Titrators, by J. M. Llabrés i Xamena, Journal of Chemical Education, Vol. 84, No. 2, 2007, pp. 325-327.
Development of a New Calibration Method for Karl Fischer Titrators, by Y. Zhang, Q. Wang, and X. Zhang, Analytical Letters, Vol. 53, No. 14, 2020, pp. 2266-2278.
Calibration and Validation of Karl Fischer Titrators: A Comparison of Different Approaches, by M. T. P. Silva, P. E. P. Ferreira, and J. L. F. C. Lima, Analytical Sciences, Vol. 29, No. 9, 2013, pp. 899-904.
A Comparison of Different Calibration Methods for Karl Fischer Titrators, by M. A. G. Zeeshan, A. Akbar, and S. G. Khan, Journal of Chemical Engineering Data, Vol. 63, No. 9, 2018, pp. 3337-3342.
Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration, ASTM D6304-16, ASTM International, West Conshohocken, PA, 2016, www.astm.org.





Please not enter any spam link in the comment box.